Innate Immune Memory Dynamics: Mechanistic Principles & Translational Applications in Inflammation Therapeutics

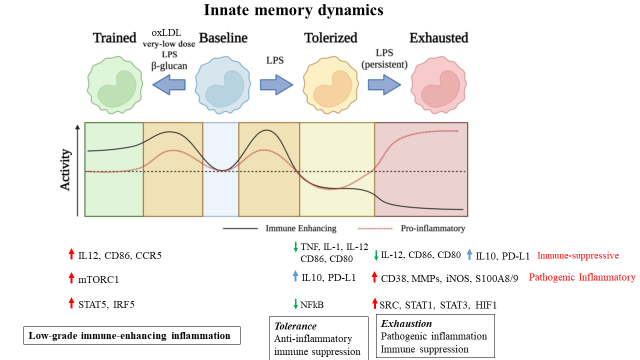
Our team studies the fundamental processes of innate immune memory and inflammation dynamics related to health and disease. We have pioneered signal-strength and duration dependent innate memory dynamics such as sustained low-grade immune-enhancing inflammation (under repetitive challenges with weak signals, e.g. subclinical endotoxemia; oxidized phospholipids; cholesterol), tolerance with anti-inflammatory immune suppression (under transient strong septic signals); exhaustion with pathogenic inflammation and immune suppression (under repetitive strong septic signals) as well as resolution of monocytes and neutrophils. Our integrated experimental and computational analyses reveal key mechanistic principles of innate immune memory. Our translational studies reveal crucial significance of distinct innate memory dynamics during the pathogenesis of diverse acute and chronic diseases such as sepsis and atherosclerosis.
Low-grade immune-enhancing inflammation memory during the pathogenesis of atherosclerosis Through integrated approaches that combine mechanistic and complementary functional examinations, we characterized monocytes with low-grade inflammatory memory features shared among mice and humans. Mechanistically, we defined the disruption of homeostatic resolution as the fundamental principle for the generation of sustained low-grade inflammation memory. We defined that the unique signaling adaptor TRAM(TICAM2) serves as an indispensable gatekeeper for initiating and sustaining low-grade inflammation memory by disrupting pexophagy. We demonstrated that the persistence of low-grade inflammatory monocytes contributes to the pathogenesis of atherosclerosis.
Innate exhaustion memory during sepsis Innate leukocytes are rewired during sepsis into a prolonged exhaustion state with the paradigm of pathogenic inflammation and immune suppression, which subjects the host to compromised defense to secondary infections as well as elevated risks for multi-organ inflammation and damage. Our group defined exhausted monocytes with the depletion of key metabolic fuel NAD+, initiated by TRAM related molecular circuitries. We first defined that exhausted monocytes can be generated by repetitive challenges with high dose endotoxin. The generation of exhaustion memory is responsible for long-term complications following the initial bout of septic insult.
Therapeutically, we are interested in developing approaches that can restore cellular homeostasis. We defined that chemical rejuvenation of peroxisome via 4-PBA or genetic deletion of TRAM adaptor can effectively reprogram innate leukocytes into active resolving states, capable of propagating innate homeostasis and reducing inflammatory disease pathogenesis. We identified resolving monocytes and neutrophils characterized by elevated CD200R and reduced TRAM expression in both murine and human systems. We characterized immune-enhancing and less pathogenic inflammatory neutrophils capable of mounting an effective and broad spectrum anti-tumor defense. Translational relevance during the pathogenesis and treatment of atherosclerosis, sepsis, cancer, and related inflammatory diseases are being examined using transgenic animal models as well as human blood samples.
Ongoing studies include biochemical, molecular as well as functional studies of innate immune memory dynamics in response to damage/danger signals with varying strength and duration under the setting of either acute or inflammatory conditions. Key intertwined players such as TRAM, TOLLIP, and IRAK-M are being examined in directing intra-cellular signaling circuitries involved in innate polarization and reprogramming dynamics. Our biochemical analyses reveal Tollip as a homeostatic molecule facilitating autophagy completion through interacting with PIP lipids. Sustained low-grade inflammatory signals disrupt Tollip function and compromises innate homeostasis.